Catalysts accelerate chemical reactions, allow reactions to be carried out under milder (and thus less energy-intensive) conditions, and enable higher selectivities and yields. High-performance catalysts thus ensure resource-saving processes, and form a central pillar of the future industrial use of renewable raw materials in the framework of green chemistry.
In the first subproject of the lighthouse project ShaPID, novel catalysts for chemical, electrochemical and biotechnological processes are being developed for application in the ShaPID demonstrators. The reactions to be catalyzed range from targeted CO2-reduction through to formic acid, the synthesis of diazo compounds and butadiene, and the fermentative (whole-cell catalytic) production of polymer building blocks.
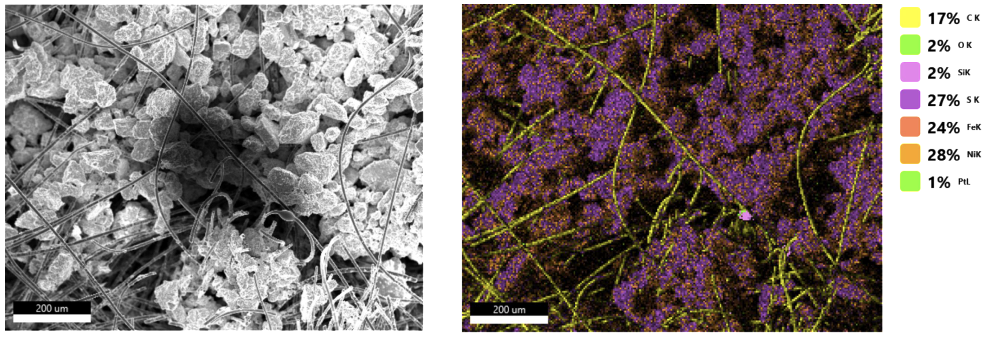
The goal of this subproject is to develop electrocatalytic, biocatalytic and chemocatalytic concepts and systems for the three ShaPID demonstrators. Research into electrocatalytic processes focuses on the direct reduction of CO2 to formic acid (or formate salts) at gas diffusion electrodes (GDEs), on the hydrogenation reactions of organic substrates and on the synthesis of organic diazo compounds or their precursors. The biocatalytic systems to be developed are whole cell catalysts, specifically bacterial production strains, which are developed into customized cell factories using metabolic engineering and systems biotechnology approaches. This enables the targeted conversion of simple and inexpensive substrates, such as formate from electrocatalytic CO2-reduction, into higher-value products. Furthermore, chemical catalysts are being developed for the synthesis of important polymer building blocks, such as butadiene, and secondary diazo products. The subproject on green catalysis technology is therefore establishing a catalytic technology portfolio which will be applied in the ShaPID demonstrators and validated for use in industrial processes.
 Fraunhofer lighthouse project »ShaPID« – Shaping the Future of Green Chemistry by Process Intensification and Digitalization
Fraunhofer lighthouse project »ShaPID« – Shaping the Future of Green Chemistry by Process Intensification and Digitalization